Rare earth fluorescence materials possess numerous advantages, including narrow emission spectral bands, high colour purity, and vibrant colours. They exhibit strong light absorption capabilities and high conversion efficiency, with emission wavelength distributions spanning a wide range. These materials demonstrate stable physical and chemical properties, high-temperature resistance, and the ability to endure high-power electron beams, high-energy radiation, and intense ultraviolet light.
These exceptional characteristics make rare earth compounds a primary focus in the exploration of advanced technological materials. Currently, rare earth materials are widely used in various applications, including lighting, displays, imaging, medical radiography, and radiation field detection and recording. This has resulted in a substantial industrial production and consumption market, with ongoing expansion into other emerging technology sectors.
Europium is a commonly used rare earth element that emits red light upon photon excitation, finding extensive applications in phosphors and laser materials. Its emission peaks are characterised by sharp spectral bands with numerous closely spaced fluorescence signals, necessitating measuring instruments with high resolution.
This Application Note studies the fluorescence characteristics of europium nitrate (Figure 1) using an Edinburgh Analytical FE30 Fluorescence Spectrometer.

Figure 1. Photoluminescent properties of europium nitrate.
Fluorescence spectra were collected using an Edinburgh Analytical Fluorescence Spectrometer (Figure 2). The emission bandwidth was changed to determine the effect of the emission bandwidth on spectral resolution.
The spectra bandwidth of a fluorescence spectrometer is a critical factor influencing its resolution. A smaller bandwidth results in higher resolution, which is essential for accurately measuring closely spaced fluorescence peaks. Conversely, a larger bandwidth increases light throughput, making it advantageous for low-signal samples that do not have stringent resolution requirements.
In cases where high resolution is not critical, utilising a wider bandwidth can enhance signal detection, thereby improving the overall analytical capability of the instrument for certain applications. The choice of bandwidth must be balanced based on the specific requirements of the analysis, optimising both resolution and signal intensity.
The emission spectrum of europium nitrate is shown in Figure 3. From this, it is evident that at an emission bandwidth of 1 nm, the fluorescence spectrum is well-characterised. The two closely spaced fluorescence peaks at 613 nm and 617 nm are well resolved.
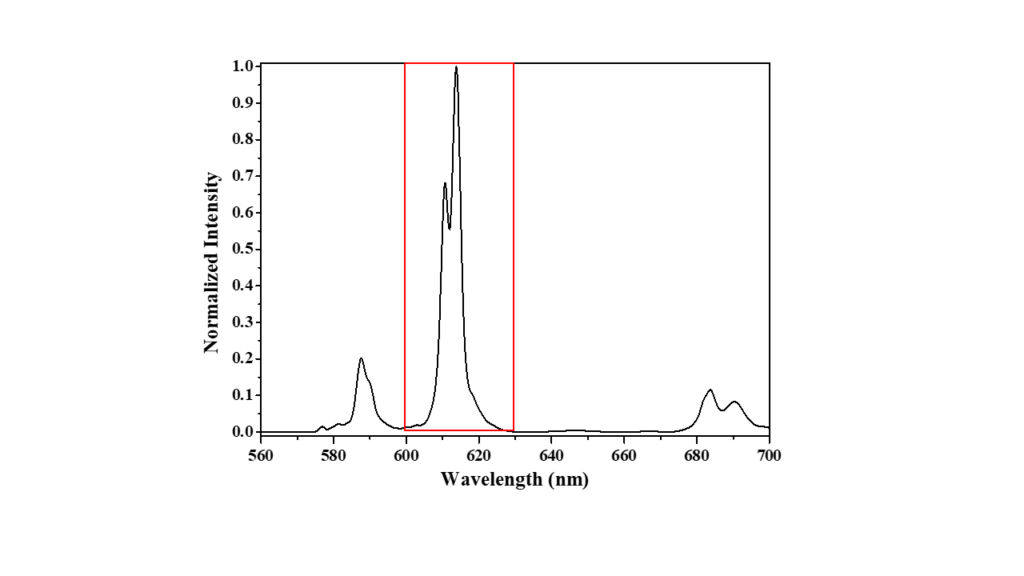
Figure 3. Fluorescence spectrum of europium nitrate at 1 nm emission bandwidth.
Figure 4 compares the fluorescence spectra of europium nitrate obtained using emission slit widths of 1.0 nm and 2.5 nm. The differences in resolution are highlighted, showing how the narrower slit width provides better separation of the emission peaks, while the wider slit width results in increased signal intensity but reduced peak resolution.
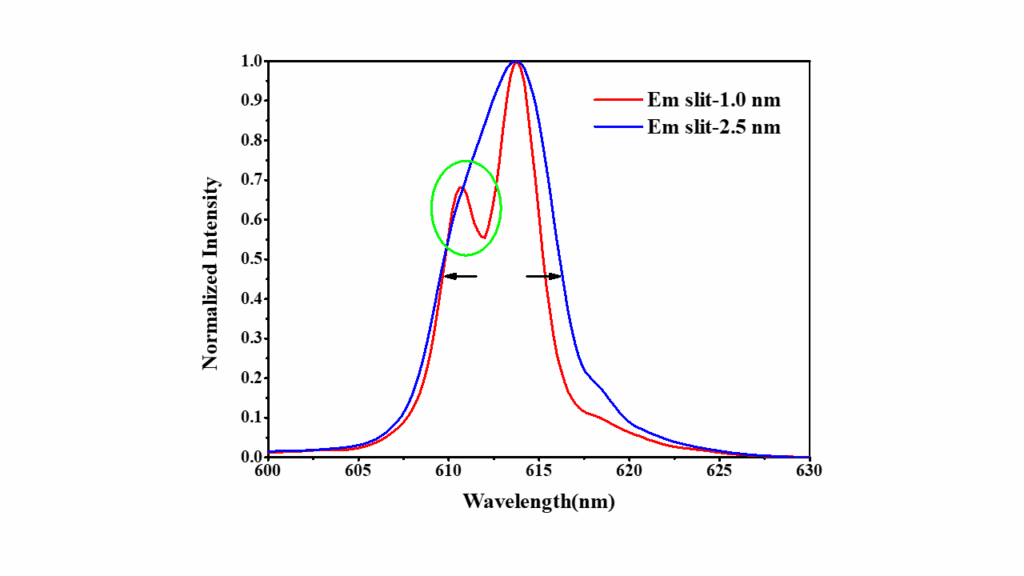
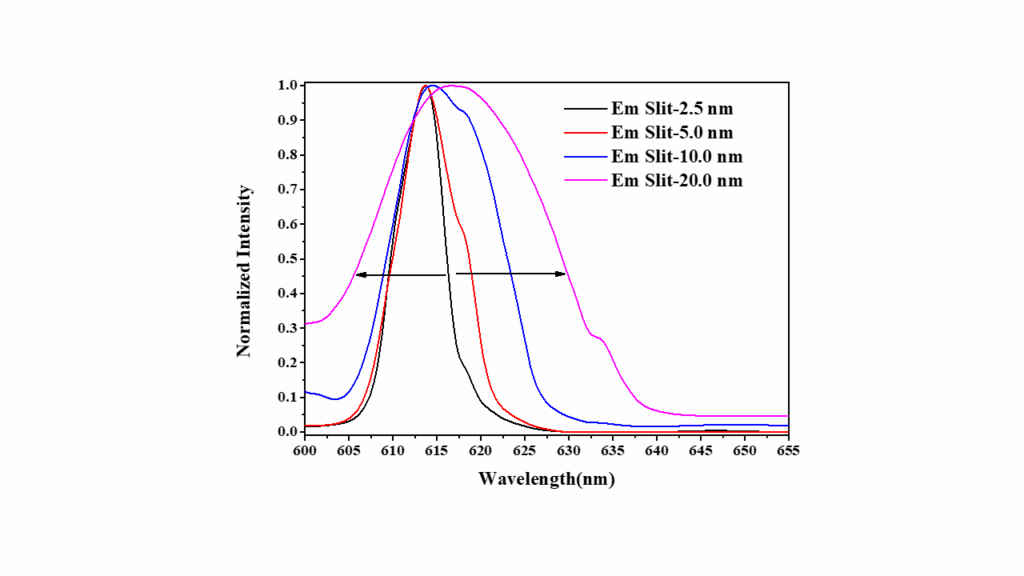
Figure 5 illustrates the fluorescence spectra of europium nitrate obtained using emission bandwidths of 2.5 nm, 5.0 nm, 10.0 nm, and 20.0 nm. As the slit width increases, the spectra show a progressive broadening of the emission peaks and a decrease in resolution. This highlights the impact of slit width on the ability to resolve closely spaced fluorescence signals.
Using a FE30 Fluorescence Spectrometer, you can optimise the signal intensity and spectral resolution with the check signal in the method development by selecting different bandwidths. This is important when analysing rare earth materials which possess sharp spectral features, such as europium nitrate.